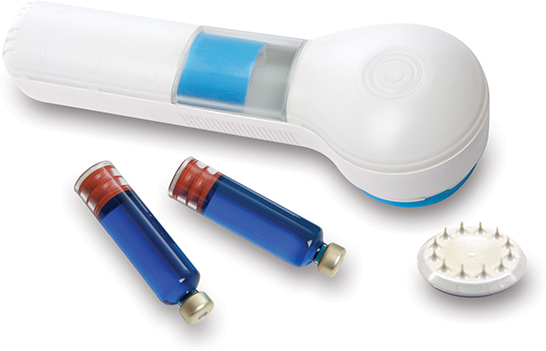
3M hollow microneedle device now available for clinical trials*; holds the potential to replace traditional injections
Getting an injection at the doctor’s office typically does not rank high on many people’s wish list. Thanks to 3M scientists’ know-how, the day is getting closer when patients may be receiving their prescription medications at home via microscopic needles. Pharmaceutical and biotechnology companies can now partner with 3M on development and conducting clinical trials using its 3M™ Hollow Microstructured Transdermal System (hMTS). Patient-friendly and easy to use, 3M hMTS is designed to open new opportunities for pharmaceutical companies and patients.
The device’s availability for clinical trials comes after conducting a number of studies and design verification tests. Based on 3M microreplication technology, pharmaceutical and biotech companies can take advantage of this patient-friendly hollow microneedle device for difficult-to-deliver biologics.
To reach this current stage of clinical readiness with the hMTS device, 3M has undertaken a rigorous process, including finalizing the device design, manufacturing critical components from medical grade materials, establishing GMP array manufacturing and device assembly, as well as filing documentation with FDA.
3M conducted a human tolerability study with the goal of selecting the appropriate microneedle array for use in clinical studies. The outcome of this study found very good delivery times for 2 mL (less than 2 minutes on average). These results provide foundational data in assessing the safety of the device. Clinical supplies are now available for assessment in potential development partners' trials.
“From the foundation laid by our recent human study, we are excited to extend our hollow microneedle device and expertise to companies who are ready for clinical studies. Pharma companies can now evaluate 3M hMTS in their clinical trials as a delivery system for a new drug product or a product line extension,” said Ingrid Blair, Vice President, Business and Marketing, 3M Drug Delivery Systems. “Keeping patient preference top of mind is key and with this new system, pharmaceutical companies have more options to satisfy patients.”
“3M™ Hollow Microstructured Transdermal System continues to demonstrate a number of unique benefits, including reproducible intradermal delivery, a proven ability to deliver formulations up to 2 mL with various viscosities, and API-dependent PK profile benefits,” continued Blair. “Its patient-friendly features and the ability for patients to easily self-administer open new opportunities to move treatments out of the clinic and into the patient’s own home. We are looking forward to working with pharmaceutical partners to provide this microneedle drug delivery alternative.”
For more information, visit 3M.com/dds or contact 1-800-643-8086.
*Disclaimer: Initiation of clinical studies may require a submission for regulatory review.
About 3M Drug Delivery Systems
3M Drug Delivery Systems partners with pharmaceutical and biotech companies to develop and manufacture pharmaceutical products using 3M's inhalation, transdermal or microneedle drug delivery technology. 3M offers a full range of feasibility, development and manufacturing capabilities to help bring products to market. Regulatory expertise, quality assurance, operations, marketed product support and other in-house resources are available for each step of the development and commercialization process. For more information, please visit www.3M.com/dds or call 1-800-643-8086.
About 3M
3M captures the spark of new ideas and transforms them into thousands of ingenious products. Our culture of creative collaboration inspires a never-ending stream of powerful technologies that make life better. 3M is the innovation company that never stops inventing. With $31 billion in sales, 3M employs 89,000 people worldwide and has operations in more than 70 countries. For more information, visit www.3M.com or follow @3MNews on Twitter.
3M is a trademark of 3M Company.
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20150128005967/en/
Karwoski & CourageMichael Gugala, 612-342-9604m.gugala@creativepr.com